Welcome to another exciting week of groundbreaking biology updates! 🌱🔍 We’re delving into some incredible advancements that have the power to transform our approach to medicine and healthcare. This week, we’re exploring AI’s role in organ development, revealing hidden mechanisms in kidney regeneration that could end reliance on dialysis, and new DNA “switches” designed to control gene expression with remarkable precision. Each discovery brings us closer to a future where biology and technology unite to tackle some of our biggest health challenges. Let’s dive into the science shaping tomorrow! 🌍💡
You can find all of the sources in this article right below each headline.
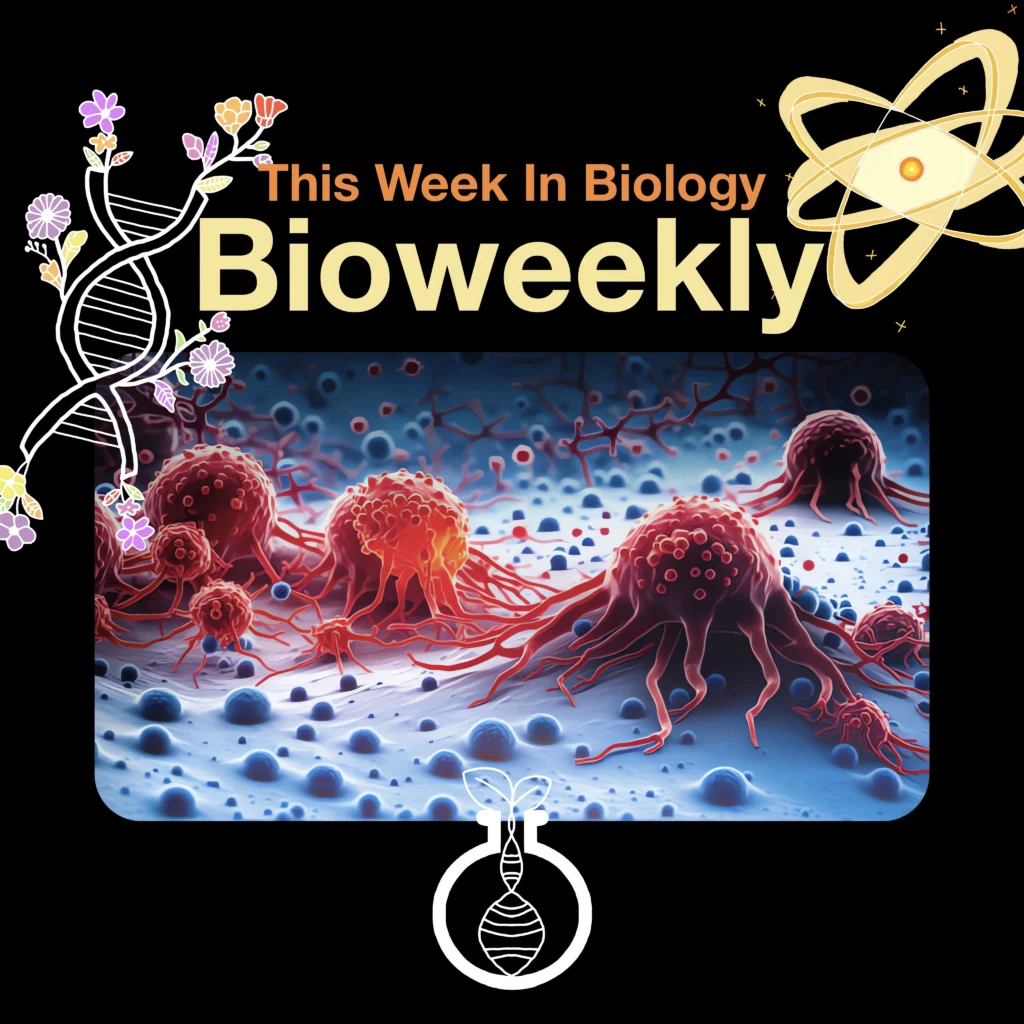
Complexity of Tumors Revealed in 3D
A groundbreaking new study by researchers at Washington University School of Medicine in St. Louis has created the first-ever 3D maps detailing the internal architecture of various tumor types. This pioneering work reveals how different types of cells within tumors — and the cells in the tumor’s surrounding environment — are organized spatially and interact with each other, providing a unique look into the physical “neighborhoods” within tumors. These findings could revolutionize our understanding of tumor biology and open up new avenues for targeted therapies.
Using high-resolution imaging, the researchers mapped six different cancer types, including breast, pancreatic, colorectal, kidney, uterine, and bile duct cancers. This study, part of the Human Tumor Atlas Network and published across the Nature journals, offers unprecedented insights into how certain “neighborhoods” of tumor cells differ in activity, gene mutations, and immune responses. For instance, the 3D maps showed that tumor cores have higher metabolic activity, fueling rapid cancer cell growth, while the outer edges exhibit more immune system activity as the body attempts to contain the tumor’s spread.
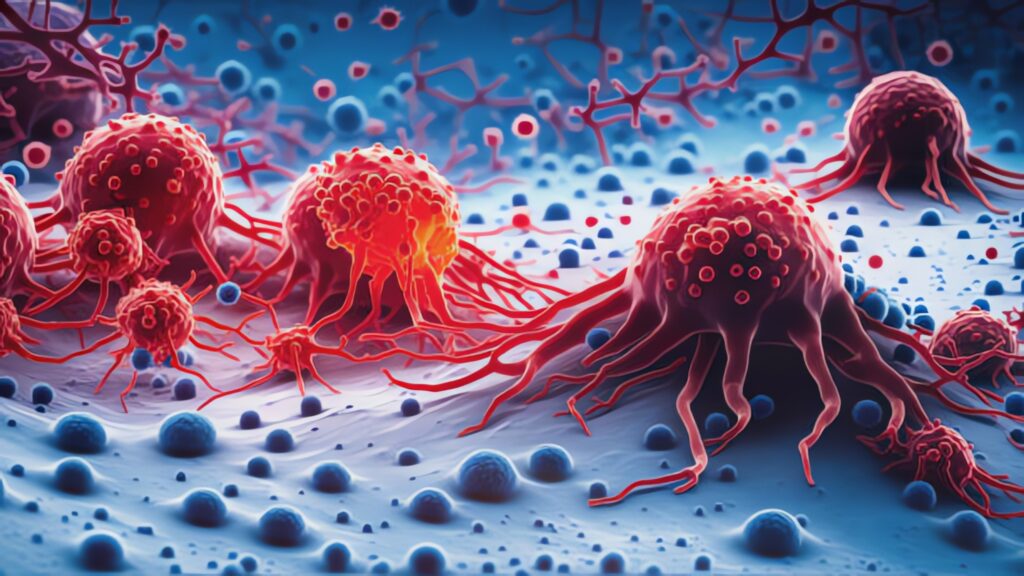
A key discovery was the identification of “hot” and “cold” immune regions within the same tumor. Hot regions, which are rich in immune cells, respond well to immunotherapies, whereas cold regions, which lack immune cells, often resist these treatments. This variability within a single tumor might explain why some cancers respond to immunotherapy initially but later develop resistance. By understanding these distinctions, researchers hope to develop therapies that can target multiple regions within a tumor simultaneously.
The study also revealed how tumors adapt to evade immune cells. In some tumors, researchers found immune cell exhaustion, a phenomenon where cancer cells overwhelm immune defenses. This exhaustion can cause immune cells to lose their effectiveness, allowing the tumor to grow unchecked. This discovery could help doctors predict which patients might benefit from treatments like checkpoint inhibitors that reactivate exhausted immune cells.
Led by Dr. Li Ding and her team, this 3D approach marks a new chapter in cancer research, showing how tumor mapping at this level of detail could help tailor treatments to a tumor’s unique structure and cellular makeup. Future work may focus on identifying specific “neighborhoods” where targeted therapies can be most effective, potentially leading to innovative treatments that tackle even the most resilient cancers.
You can find the full article from here
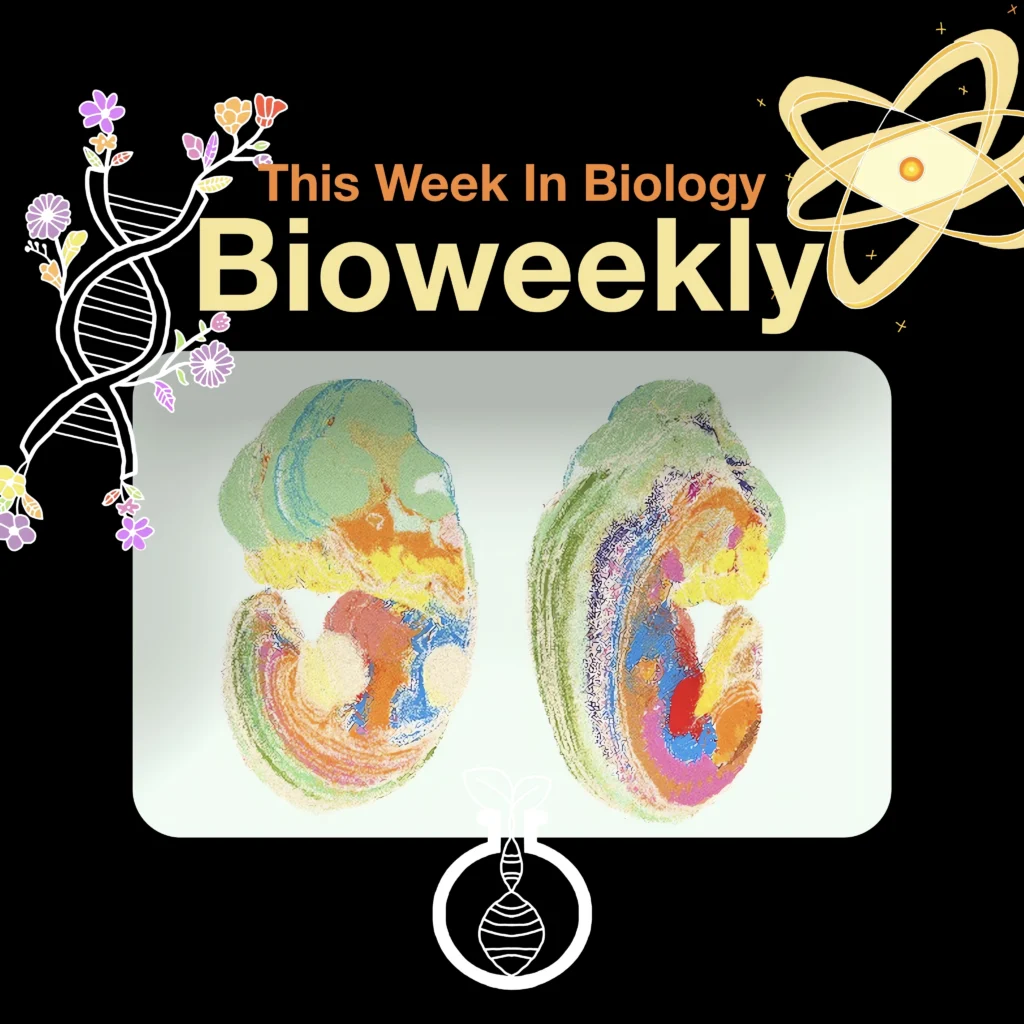
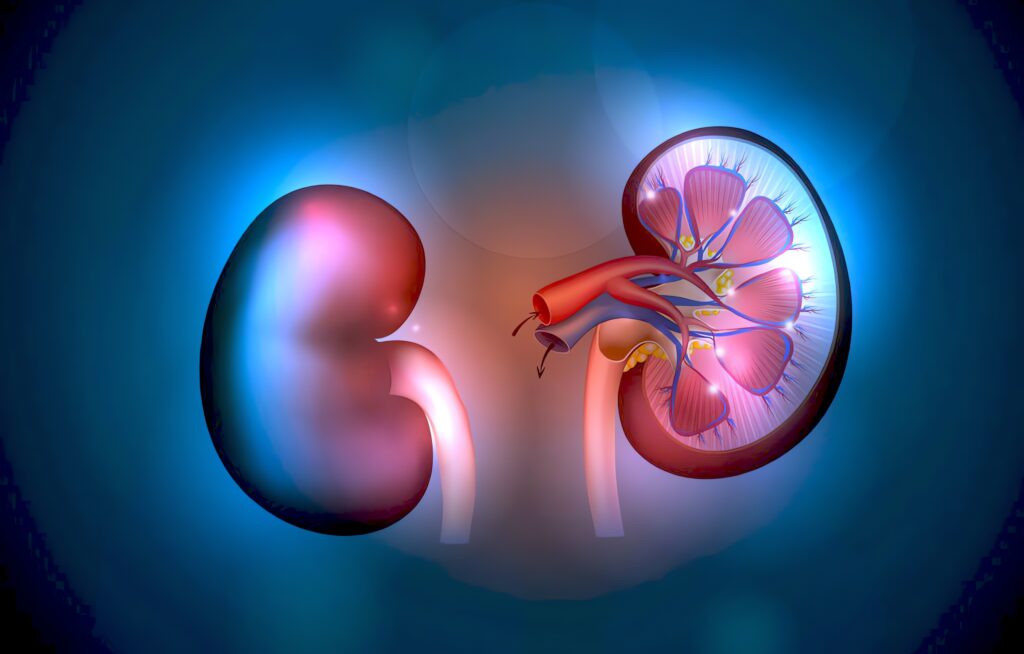
Developing Kidneys from Scratch
A groundbreaking study led by Alex Hughes, Assistant Professor in Bioengineering at Penn Engineering, has shed light on kidney development, uncovering new mechanisms and techniques that could pave the way toward lab-grown kidney tissues, reducing reliance on dialysis and transplants.
Kidneys are intricate organs densely packed with nephrons—tiny units that filter waste from the blood. Over time, however, lifestyle factors and limited regenerative capacity lead to a high burden of chronic kidney disease (CKD), impacting over 850 million people worldwide. Hughes and his lab aim to recreate the kidney’s unique structure by understanding the biological “blueprint” that guides kidney growth.
The team’s research dives into how kidney tubules, arranged like tiny branching forests, respond to mechanical stress waves—a process compared to the push-and-pull of crowded elevators. These stress pulses appear to prompt the formation of nephrons in a highly organized, almost choreographed manner. This insight offers clues to guiding the growth of artificial kidney tissues with the complex arrangement necessary for kidney function.
With kidney disease on the rise and the demand for transplant organs far exceeding supply, these advancements represent a promising leap toward regenerative medicine.
You can find the full article from here
Researchers Flip Genes On and Off with AI-Designed DNA Switches
Scientists have used AI to create thousands of synthetic DNA “switches” that precisely control gene expression in specific cell types. This breakthrough, developed by teams at JAX, the Broad Institute, and Yale, could lead to advancements in gene therapy and medical research by allowing scientists to turn genes on or off in select tissues—like activating a gene in liver cells but not in blood or brain cells. Their tool, CODA (Computational Optimization of DNA Activity), allows precise DNA switch designs to fine-tune gene activity in specific cells, making it possible to “pick and choose” where genes are active in the body. This revolutionary method, which has already shown promising results in cells and animal models, may one day transform therapies by providing unparalleled control over gene expression in target organs, holding promise for fields like biomanufacturing and personalized medicine.
- New AI-Driven CREs: These AI-designed synthetic switches, known as cis-regulatory elements (CREs), offer unmatched specificity, activating genes only in the desired tissue.
- Precision Control in Animals: In lab tests, certain synthetic CREs activated specific proteins only in the liver of developing zebrafish, demonstrating their high precision.
- Potential for Medical Innovation: This method could redefine how gene therapies are developed, targeting only affected tissues and minimizing potential side effects, especially crucial for personalized medicine.
You can find the full article from here
Thank you for diving into this week’s news with us. We hope you enjoyed uncovering these fascinating updates as much as we did. Be sure to return next week for more exciting discoveries from the world of science. Until then, stay curious and keep exploring!


My name is Ali Emre Cabadak, a dedicated biology enthusiast currently pursuing my studies at Marmara University, where I am majoring in Bioengineering. As a passionate advocate for scientific discovery and innovation, I am the founder of Biologyto. My goal is to bring the wonders of biology closer to everyone and inspire a new generation of thinkers and innovators. Through Biologyto, I aim to write scientific articles that delve into the fascinating world of biology, sharing insights and discoveries that inspire curiosity and innovation.